Anyone who has ever worked in a vineyard understands that Botrytis cinerea (grey mold) is a serious threat to the health and quality of the grapes. This mold is responsible for huge crop losses in many parts of the world, and is responsible for billions of dollars in damage in the United States and all over the globe. Wine quality from botrytised grapes is often reduced, due to moldy, mushroom-like, and rotten smells. Botrytis cinerea infection can result in desirable wine, however, when it is present in some white varieties that are used to produce sweeter late harvest wines (“noble rot”).
 |
| http://1.bp.blogspot.com/_KMlT-iVv-b0/ SbI_ixDtnzI/AAAAAAAABlw/ OJSvIwfeTrc/s400/noble-rot.jpg |
Early on in the spring, B. cinerea can infect the leaves of the grapevine, spreading throughout the leaves and flowers. In the summer, the mold can infect the grapes themselves, the success of which depends on many factors, including the genetic structure of the B. cinerea, climatic conditions, the architecture of the grape cluster, and the susceptibility of the berry. Infections can occur by a couple of different mechanisms, including airborne attacks by spores onto open wounds in the berry caused, for example, by hail or insect damage; and also infections spread by fungal vegetative filaments from one berry to the next.
Controlling B. cinerea in vineyards can be done several ways, though often requires treatment of fungicides, though it can also be controlled by changing vineyard management/cultivation practices. The mold is often detected by patrolling the vineyards, using ELISA techniques (Enzyme Linked Immunosorbent assay), forecasting utilizing climatic data, and finally at harvest, botrytised grapes can be sorted to remove infected grapes.
Once entered into the grape, B. cinerea induces defensive reactions in the plant. The primary mechanism is the synthesis of phytoalexins and pathogenesis-related proteins, including resveratrol and similar stilbenic compounds. Resveratrol, which has been extensively studied as a compound that is very beneficial for human health, can easily be seen around B. cinerea infection sites due to its blue florescence in grape leaves and fruit. Mechanistically, it is assumed that B. cinerea detoxifies stilbenic compounds (resveratrol and pterostilebene, a highly toxic compound for fungi) using laccase phenol-oxidizing enzymes in order to colonize the plant. At the same time, there is a decrease in chlorophyll concentrations in leaves of grapevines infected by B. cinerea, which induces the plant to increase photosynthetic activity in the leaf surrounding the infection site, which could also be detectable using plant fluorescence.
Due to the ability of many of the aforementioned compounds to fluoresce in grapes and grape vines, the authors of the study presented today hypothesized that is would be possible to detect B. cinerea infections on grapes using remotely sensed fluorescent techniques. This sort of technology has already been used for detecting fungal infections in other plant species, where researchers have found increases two different fluorescence ratios (instead of one wavelength) during the active infection. Therefore, it was the goal of the study presented today to evaluate the autofluorescence response of grapes to Botrytis cinerea infection under controlled conditions, and to determine if earlier detection of the infection is possible using remote sensed technology.
Methods
Grape clusters of the white cultivar Italia of 500, 550, and 660g were used in three separate experiments (A, B, and C). For each cluster, 10 berries were randomly selected, detached from the bunch, and surface sterilized by immersion into 95% denatured ethyl alcohol for 30 seconds.
The strain of Botrytis cinerea used was 234 (transposa genetic type, Group-II), since it is a more aggressive strain than others. The mold was originally collected in 1998 at harvest in a Bordeaux vineyard from Sémillon blanc grapes and grown in petri dishes on 1.5% malt agar solid medium.
To inoculate the grapes with the mold, mycelial plugs of 4mm in diameter were taken from these media. The skin of all berries was pierced with a fire-sterilized needle, with one plug per berry placed at the site skin piercing. For Experiment A, 5 berries were inoculated on October 10th, 5 on October 18th for Experiment B, and for Experiment C, 5 berries were inoculated on November 26th, 2006.
For each of the three experiments, controls were created by inoculating 5 berries with a malt agar disc without the mold at the same skin piercing point on the berry. Inoculated berries were kept in a humidity-controlled chamber. The incubation period for each experiment was the time between inoculation and the first fluorescence measurements: 3 days for Experiment A; 2 days for Experiment B; and 0.25 days for Experiment C.
Fluorescence measurements were taken at 3 and 6 days after inoculation for Experiment A, 2 and 5 days for Experiment B, and 0.25, 1, 4, and 6 days for Experiment C. Measurements were taken via an imaging fluorometer using an interline charged-coupled device camera modified for on-chip accumulation capability.
In addition to fluorescence measurements, images were created and analyzed. For each image, a mask was manually delineated in order to determine the berry area and to exclude non-berry pixels for data analysis. Fluorescence ratios for each berry were also calculated: F440/F520, F440/F690, F440/F740, F520/F690, F520/F740, and F690/F740.
After calculating the fluorescence ratios and creation of the masks, spatial average was calculated for each berry, with one value per berry for each ratio and each date of measurement.
Due to strong fluorescence of the agar plugs, they were removed right before the first day of measurements for each experiment.
Results
- Due to the removal of the agar plug right before the first fluorescence measurement, visible development rate of B. cinerea was influenced by the inoculation period.
o For Experiment C, plugs were left in for only 6 hours, therefore colonization of B. cinerea was slower than for Experiments A and B.
- Experiment A, 6 days after inoculation: all 5 berries expressed symptoms of grey mold infection.
- Experiment B, 5 days after inoculation: 4 out of 5 berries expressed symptoms of grey mold infection.
- Experiment C, 4 days after inoculation: all 5 berries expressed symptoms of grey mold infection.
- In the digital images:
o Control berries showed a circular scar that was darker and slightly larger than the needle diameter.
o Infected berries showed a brown rotted lesion surrounding a split which was leaking interior contents of the grape.
- Images under UV light:
o There was a strong blue fluorescence (due to the presence of resveratrol) around the contaminated area on the infected berries. The fluorescence distribution was associated with the spreading lesion on the berry.
o The infected area was surrounded by a band of fluorescence in what looked like a healthy area of the grape up to 5mm in front of the visibly noticeable infected area.
o In control berries, blue fluorescence was clearly detectable, though restricted to the needle wound site.
§ Since fluorescence was noticed on both infected and control berries, determination of a Botrytis cinerea infection solely based on these digital images could be confusing and potentially lead to incorrect diagnoses.
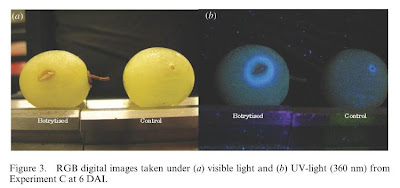 |
| Figure 3 from Belanger et al, 2011 (DAI = days after inoculation) |
- For some of the fluorescence ratios, there were significant differences between infected and control berries.
o The best ratio for early detection of B. cinerea infection was F440/F740, which increased significantly in infected berries than control berries.
- Fluorescence was seen on not only the infected berries but the control berries as well, which was possibly due to the healing process at the needle site, which involves lignification and accumulation of phenolics at the wound site.
o Mechanical wounding activates the same genes that are involved with healing and defense against pathogens in plants.
- The fluorescent area was constant with control berries, while was variable and evolving with infected berries.
o The central area within the developing lesion in the infected berries no longer fluoresced at 3, 4, or 6 days after inoculation.
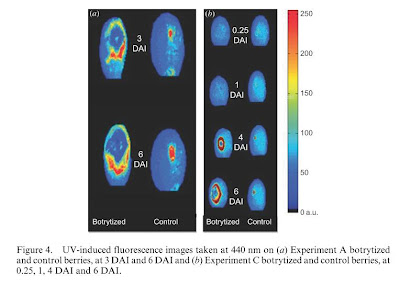 |
| Figure 4 from Belanger et al, 2011 (DAI = days after inoculation) |
o The boundary of the lesion showed a higher F440 intensity.
§ This is possibly due to the fact that B. cinerea produces enzymes (laccases) that digest plant phenolics (stilbenes in particular) that were originally present in the developing lesion. As the lesion expands, stilbenes and other phenolics are recruited to the site, while behind the front laccases and other enzymes produced by the mold digest and destroy them.
o The fluorescence at 440nm very clearly showed colonization by B. cinerea, however in the early stages of infection before 3 days after initial inoculation, it is too difficult to distinguish between B. cinerea infection and a simple skin wound such as hail damage or an insect prick.
- Using edge detection via UV-epidermal transmittance, it was possible to detect the B. cinerea infection area without detecting a simple mechanical injury as with the control berries.
o There was no fluorescence detectable on the control berries.
o Infected berries showed significantly more edges detected than control berries.
§ These differences were detected as early as 3 days after inoculation for Experiment A, and 0.25 days after inoculation for Experiment C.
§ For infected berries, the colonized area by B. cinerea at the surface of the berry was detected by an increase in epidermal transmittance at 690nm, which resulted in higher proportions of edges detected.
· These results were hypothesized to occur as a result of laccase being secreted by B. cinerea during the infection process catalyzing the UV-absorbing components of the grapes, which would therefore increase the amount of UV light reaching the chlorophyll in the berries and increasing the epidermal transmittance.
Conclusions
Studies have found that initial fungal colonization of plants result in an increased concentration of resveratrol, a compound that is toxic to fungi, to the point of infection in order to attempt to prevent further spread by the pathogen. As a result of this influx of resveratrol (and other phenolics), there is a notable increase in fluorescence at 440nm, which is focused mainly on the infection/colonization front. If B. cinerea overcomes the initial flux of resveratrol, it overpowers the defensive system of the berries and completely colonizes the grape by catalyzing the resveratrol and other phenolics by action of the phenol-destroying enzyme laccase. The destruction of the resveratrol and other phenols results in a decrease in UV light absorption and a decrease in the fluorescence at 440nm. Therefore, when looking at digital images under UV light, there is a blue fluorescence at the colonization front, whereas there is no fluorescence in the colonization center where the fluorescing compounds have been digested and destroyed by the B. cinerea enzymes.
Even though fluorescence of resveratrol and other stilbenes were detectable under UV light at 440nm, mechanical wounding caused by the needle prick (and therefore similar to insect bites or hail damage, etc), it could get confusing determining whether or not a grape is actually infected with B. cinerea or if it’s simply banged up a little by looking at digital images of one wavelength (in this case, 440nm) alone. However, the results of this study did show that if one looked at the ratio of two different wavelengths, specifically F440/F740, one would be able to distinguish between berries infected with B. cinerea and berries that are healthy or only slightly damaged by simple mechanical means. This study also found that using this ratio method, one could detect B. cinerea infection as early as 4 days after inoculation.
Based on these results, the authors suggest a possible hand-held device that can easily measure fluorescence absorbance at the 440nm and 740nm wavelengths in the field and calculate a ratio for determining infection status of the grapes. This sort of device could be implicated during several different points of the year, including in the vineyard during the growing season to help improve defensive control strategies and during the sorting process after harvest in order to better select healthy grapes versus infected grapes. A critical threshold value for this ratio would need to be determined through experimentation, though the authors of the current study suggest a critical value between 0.8 and 1.0 based on their own data, though more work under varying conditions should be done before this value is accepted as standard.
The results of this study provide important information for developing a technique for identifying Botrytis cinerea infected grapes earlier than current methods, which could aid in improving treatment implication times and sorting. More work needs to be done to gain more knowledge on the possibility of using UV fluorescence ratios for early detection (as early as 3 days according to this study), but this is a solid step in the right direction.
I’d love to hear what you all think about this topic. Please feel free to comment below (no html tags, please).
Source: Bélanger, M.C., Roger, J.M., Cartolaro, P., and Fermaud, M. 2011. Autofluorescence of grape berries following Botrytis cinerea infection. International Journal of Remote Sensing 32(14): 3835-3849.
DOI: 10.1080/01431161003782064
I am not a health professional, nor do I pretend to be. Please consult your doctor before altering your alcohol consumption habits. Do not consume alcohol if you are under the age of 21. Do not drink and drive. Enjoy responsibly!

4 comments for “Using UV-fluorescence to Detect Grey Mold (Botrytis cinerea) Infections: Possible Implications for Field Detection Technologies”