Often times, scientific research articles have implications for applications into the real world—for example, on Monday, I posted an article on examining 2D-COS as a possible screening method for smoke taint in wine. The results of the study had significant implications for the wine industry, mostly in terms of time and money savings for the winery.
Other times, scientific research articles take a look at something simply for science’s sake. Today’s article is one of those papers. Sure, one could probably finagle a way to justify why the research was done in terms of real-world applications, but mostly I feel as though this research is more “for fun” or “just because”.
The purpose of the study presented today was to examine the physics behind a champagne cork popping out of a bottle, and how temperature influences that
![Photo By Niels Noordhoek (Own work) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons](http://www.academicwino.com/wp-content/uploads/2013/04/Champagne_pop_The_Academic_Wino-199x300.jpg)
Photo By Niels Noordhoek (Own work) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Most people reading this blog know that champagne/sparkling wine bottles are under pressure due to the contents inside. Under the traditional method, secondary fermentation is performed inside of a sealed bottle, thus trapping the CO2 produced by the yeasts inside as tiny bubbles. In a standard sized champagne/sparkling wine, there are approximately 12g/L of dissolved CO2 molecules present after secondary fermentation is complete, which equals about 5L of gaseous CO2 under standard temperature and pressure. After disgorging (i.e. removal of caps to get rid of the dead yeasts and other sediment that collected) and re-corking, dissolved CO2 content re-equilibrates to a value of about 11g/L.
According to basic physics and chemistry principles, the solubility of CO2 into wine is highly dependent upon the temperature: specifically; the lower the temperature, the higher the solubility of CO2. Since partial pressure of a gas in a closed system is linked to solubility in the system, the partial pressure can be assumed to be highly dependent upon temperature as well. Finally, since the force of a champagne cork popping out of a bottle is dependent upon how much pressure is behind it, one can also assume that the force of a champagne cork popping out of a bottle is dependent upon temperature.
I suppose one real-world application of this type of study would be in determining the proper temperature of popping the cork of a bottle of champagne for reduced risk of popping one’s eye out with the flying cork. The article presented today cited some statistics from the American Academy of Opthalmology which claimed that champagne cork-popping is responsible for the most common eye injuries at holiday time. Even though the proper way to open a bottle of champagne/sparkling wine is to slowly remove the cork with a gentle hiss, but since tradition calls for dramatic antics, a lot of people prefer to have the cork going flying in a spirit of celebration. Thus, the results of this study may provide information on the proper temperature of a bottle of champagne/sparkling wine for lowest risk of eye injury due to excessive cork-popping forces (side note: it was really hard to write that sentence without laughing at how ridiculous it all sounds).
My favorite part of this paper was when the authors made a little aside regarding the “Father of Champagne” Dom Pérignon. Dom Pérignon is often credited with inventing champagne (some may argue against this, but that’s not the point of this story) as well as developing efficient champagne cork stoppers. The authors said that is was “worth noting” that Dom was blind by the end of his life, implying that maybe he poked his eyes out too many times while developing efficient champagne corks, but alas, they followed up by reporting that he was actually blind for reasons other than champagne cork-popage, but apparently that fact was funny to them nonetheless.
But I digress…..
To recap, the goal of this study was to examine the physical properties of a champagne cork popping out of a bottle of champagne at 3 different temperatures using high-speed infrared imaging technology.
Methods
Commercial Champagne wine from the Cooperative Nogent l’Abbesse in Marne, France (2008 vintage) was used for this experiment.
Bottles were of standard size (75cL) and contained about 9g of CO2 per bottle (equivalent to 12g/L).
Wines were aged for 15 months then disgorged and closed with a natural cork stopper (part agglomerated, part solid cork). The volume of the head space after corking was 25 mL.
Wines were stored at 12oC until ready for use.
24 hours prior to the experiment, wines were stored at and equilibrated to 3 different temperatures: 4, 12, and 18oC.
Champagne bottles were popped and physical parameters were measured using techniques based on infrared thermography principles. To visualize CO2, cameras were equipped with a band-pass filter focused on the CO2 emission peak. The camera took images at a rate of 100 frames per second. The shutter speed on the camera was 1ms.
For each temperature, champagne bottles were popped in replicates of 3 to calculate an average velocity of the cork flying.
Results
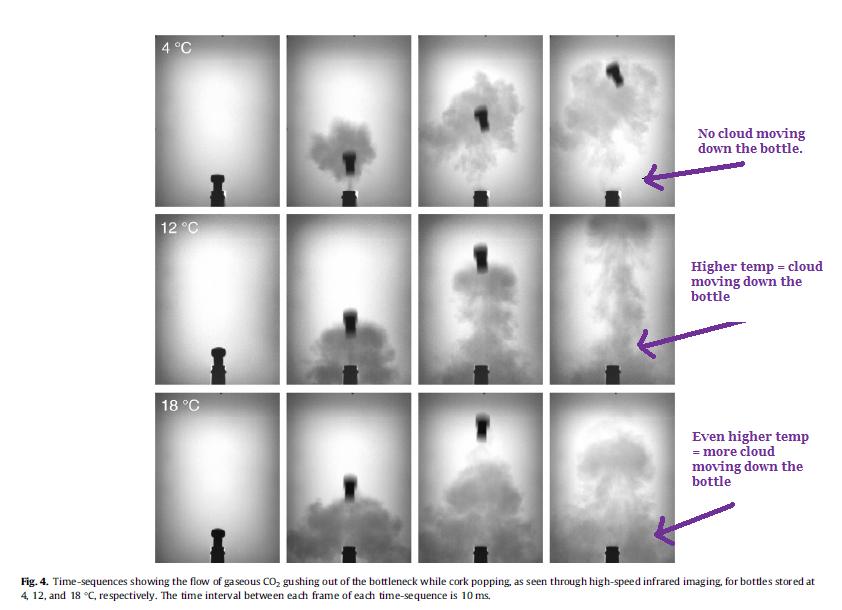
• The lower the champagne temperature, the smaller the cloud of CO2 that escapes from the bottle after popping the cork.
o In other words, the volume of the CO2 cloud after cork popping increases with increasing temperature.
• The higher the temperature, the more the CO2 cloud moves down the bottle neck (see Figure 4 from Liger-Belair et al, 2013).
• The expansion of the CO2 gas after being released from the champagne bottle after cork popping corresponds with a sudden decrease in temperature of that gaseous cloud.
o The higher the champagne temperature, the lower the temperature reached by the expanding CO2 gas cloud after cork popping.
o The lower the temperature of the CO2, the higher the density: thus a possible explanation for why at higher temperatures, the CO2 cloud travels down the bottle neck more than a lower temperature wine.
• The cloud of “fog” noticed in the visible spectrum (i.e. to the naked eye) is due to the sudden drop of temperature in the expanding CO2 gas cloud and subsequent condensation of water vapor and ethanol vapor.
o The “fog” is NOT CO2.
• Average cork velocity increased with increased champagne temperature.
• Only a small fraction (5%, regardless of the champagne’s total temperature) of the total energy released from the cork popping process was converted into kinetic energy (i.e. energy due to motion).
Conclusions
The results of this study were pretty interesting from a pure physics standpoint. It was neat to see how increasing champagne temperature changed the structure of the CO2 cloud, as well as how it increased the velocity of the cork flying out. I suppose if one were to try and reduce the risk of eye injury due to champagne cork impalements, I would suggest making sure the temperature of the wine was as cold as possible.
Also interesting from a pure physics standpoint was the fact that only 5% of the champagne’s total temperature was actually converted into kinetic energy. That means there is a ton of energy not accounted for in this study. Where does that energy all go if only 5% of it goes into the physical motion of the cork? The authors suggest, and I tend to agree, that the majority of the energy in the cork popping process is transferred to sound energy—i.e., the sound of the loud cork POP! Further tests would be needed to determine how much energy is converted to sound energy, and how any remaining energy is converted.
I don’t particularly care if this study doesn’t really contribute much to the advancement of the wine industry—sometimes it’s just fun to learn a little bit of random information about something you like!
In addition to reducing eye injuries, this study could have potential implications for the development of better corks, cages, or even champagne bottles, but certainly there are no conclusions about any of this that can be made based on the particular experiments performed.
What do you all think of this study? Please feel free to leave any comments!
Source: Liger-Belair, G., Bourget, M., Cilindre, C., Pron, H., and Polidori, G. 2013. Champagne cork popping revisited through high-speed infrared imaging: The role of temperature. Journal of Food Engineering 116: 78-85.

5 comments for “The Physics of The Champagne POP!: How Temperature Influences Cork Velocity and Gas Cloud Kinetics”