Phylloxera (Daktulosphaira vitifoliae Fitch) is one of the most destructive pests for grape vines. Historically, it has wreaked havoc on vineyards around the globe, and nearly wiped out the entire grape and wine industry in Europe (and many other parts of the globe) starting in the 19th century. (Side note: for a comprehensive historical account of the phylloxera crisis, check out this book that I reviewed earlier this year: Dying on the Vine: How Phylloxera Transformed Wine by George Gale).
There is no one method that is adopted globally to completely eradicate phylloxera, however, there are two main approaches that are used to attempt to keep the pest at bay: 1) grafting Vitis vinifera vines on the phylloxera-resistant American rootstock; and 2) quarantine methods to reduce the spread of phylloxera into non-infested areas. Most viticulture areas around the globe employ the rootstock method, however, in Australia; the quarantine method is the method of choice. The remainder of this post will focus on phylloxera quarantine and eradication methods in the Australian wine industry.
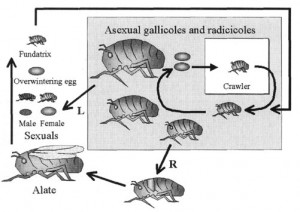
Photo from the Pests and Diseases Image Library:
http://old.padil.gov.au/pbt/files/uall/GP_lifecycle_from_Granett_et_al_2001_paper.jpg
Previous studies have found that phylloxera most often disperses to other vines and vineyards when it is in the first instar nymph life stage. These are basically the creepy crawly baby phylloxera that has not yet matured into winged adults (in the image, see the white “crawler” highlighted critter). Phylloxera at this stage is often in the vines and the leaves, and the risk for transferring these pests from one vineyard to another is highest during the spring and summer months when vineyard machinery is moving back and forth during their usual vineyard management programs. These pests are tough little creatures, and are known to survive a lot of intense handling, including being squashed in between grape bins, and also the crushing, de-stemming, and pressing methods after harvest.
To minimize the transfer of phylloxera from infested to non-infested vineyards in Australia, strict quarantine methods are employed. These methods include regulations for movement of machinery procedures, hygiene and cleaning procedures, and specific quarantine requirements. Specifically, in Australia, there is a dry heat treatment requirement for all vineyard machinery which states that machinery needs to be kept in a temperature-controlled room at 45oC for at least 75 minutes or at 40oC for 120 minutes.

By Ji-Elle (Own work) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Previous studies have shown that increased temperatures have an effect on phylloxera survival and development, though these studies more often focused on only one type of phylloxera (there are 83 known genotypes of the bug) or one geographical location. Though there are 83 different genotypes, there are two that are particularly widespread and virulent throughout Australian vineyards: “G1” is the phylloxera strain that is most widespread in Australia, and “G4” is the strain that is the most dangerous/harmful, and according to some are the two strains that provide the greatest threat to the Australian grape and wine industry.
The goals of the study presented today were to the minimum amount of time required for 100% mortality in phylloxera under different temperature and humidity conditions, to compare the impact of these conditions on the mortality of the G1 and G4 strains of phylloxera, and to confirm whether or not the current quarantine protocol for Australian vineyard machinery is effective in reducing the chance of further phylloxera infestations.
Methods
G1 and G4 strains of phylloxera were obtained from invested vineyards in the Yarra Valley and King Valley, Victoria, Australia. Insects were confirmed to be one or the other strain by DNA microsatellite marker analysis. Phylloxera were kept on Vitis vinifera roots until ready for use. Eggs were incubated at a constant temperature for 5-7 days, and then newly hatched first instars of phylloxera were collected and used for the experiments.
Environmental chambers were created to maintain a constant temperature and humidity level. Temperatures in chambers were kept between 30 and 45oC at 5oC increments with humidity levels at either 30% or 100%. Separate chambers were used for each temperature and humidity combination. 20 newly hatched first instar phylloxera were placed in a vial with mesh on the ends to 1) prevent escape and 2) maintain air flow. 5 replicates of each vial were placed in each environmental chamber (100 instars per environmental chamber).
Instar survival was measured using a low-powered binocular microscope every 15 minutes for 30-120 minutes, depending upon the treatment.
Results
· The highest temperature found to achieve 100% phylloxera mortality was 45oC regardless of humidity level.
o The G1 strain exposed to 45oC achieved 100% mortality by 75 minutes at 30% humidity and by 90 minutes at 100% humidity.
o The G4 strain exposed to 45oC achieved 100% mortality by 75 minutes at 30% humidity and by 60 minutes at 100% humidity.
· At the 40oC temperature, 100% mortality was achieved at the 30% humidity level.
o The G1 strain required 90 minutes to reach 100% mortality under these conditions.
o The G4 strain required 105 minutes to reach 100% mortality under these conditions.
· At 40oC and 100% relative humidity, 4% of the G1 strain survived and 7% of the G4 strain survived after an exposure time of 2 hours.
· No other combination of temperature and humidity achieved 100% mortality for either phylloxera strain.
· At 30oC and 30% relative humidity, survival was 60% for the G1 strain and 70% for the G4 strain.
· At 35oC and 30% relative humidity, survival was 30% for both the G1 and G4 strains.
· Temperature showed a significant effect on mortality of phylloxera.
· Humidity did not have a significant effect on mortality of phylloxera.
o The combined effect of temperature and humidity had a significant effect on mortality of phylloxera (though humidity alone had no effect).
· There was no significant difference between the mortality rates of G1 and G4 strains of phylloxera at the different temperature and humidity combinations.
Conclusions
According to the authors, this is the first study that examined and showed the amount of time required to achieve complete mortality in first instars of

Photo attained from: http://upload.wikimedia.org/wikipedia/commons/thumb/a/a8/Phylloxera_cartoon.png/615px-Phylloxera_cartoon.png
phylloxera at different temperatures and humidity combinations (30oC, 35oC, 40oC, and 45oC; 30% and 100% relative humidity). They were also able to confirm that the current quarantine protocol set forth by the National Vine Health Steering Committee in Australia of 45oC for 75 minutes exposure time or 40oC for 120 minutes exposure time are effective against the proliferation of phylloxera in non-infected areas.
The authors noted that with increased relatively humidity, the survival rate of the phylloxera instars went up. They mentioned that this result shouldn’t be too worrisome, as the relative humidity in these designated “dry rooms” should not get much higher than 50%. More work should be done to examine the survival rates of phylloxera at relative humidity between 30% and 50%, as these levels would be more likely to occur in one of these rooms than a relatively humidity of 100% as was tested in this study. These results may determine if the regulations of in-room humidity control need to be strengthened or not.
It would also be interesting to see how other strains/genotypes of phylloxera survive under similar conditions. Do the other 81 known strains have similar survival rates as G1 and G4? Or are there some particularly tough strains that could take over once the competition was eradicated? Even if those strains aren’t so common, they could be if they ever attained a competitive advantage. Research into how to effectively quarantine these strains may be valuable for these reasons.
In general, the results of this study provide strong support for the procedures implemented currently for the quarantining of vineyard machinery in attempts to reduce or eliminate the spread of phylloxera into non-infested areas. Further research could shed some light onto whether or not these methods may be effective in other locations around the world and may add an extra level of defense against the ever-present vineyard-destroying pest.
I’d love to hear what you all think about this research and the topic in general. Please feel free to leave your comments!
Source: Korosi, G.A., Mee, P.T., and Powell, K.S. 2012. Influence of temperature and humidity on mortality of grapevine phylloxera Daktulosphaira vitifoliae clonal lineages: a scientific validation of a disinfection procedure for viticultural machinery. Australian Journal of Grape and Wine Research 18: 43-47.
